How to Construct a Galvanic Cell
R s R aq S aq S s which is the cell diagram for this galvanic voltaic cell. In this project you will make different galvanic cells and connect them to flashlight lamp to test.

Galvanic Cell Or Voltaic Cell Construction And Working Galvanic Cell Chemistry Study Guide Science Chemistry
One of these electrodes the cathode shall be a positively charged electrode while the other shall be the anode the negatively charged electrode.
. It may be represented as shown in Fig. The cell reaction is of redox kind. Youll then identify unknown metal electrodes using known standard potentials and determine the magnitude of the voltage produced.
In these devices the Gibbs energy of the spontaneous redox reaction is converted into electrical work that can be used to drive a motor or to power electrical equipment such as heaters fans. The key to gathering the electron flow is to separate the oxidation and reduction half-reactions connecting them by a wire so that the electrons must flow through that wire. 2Ag aq Ni s --- 2Ag s Ni2 aq A galvanic cell has two solutions.
A galvanic cell is constructed in which an AgAg half cell is connected to a Ni2Ni half cell. Setting Up Galvanic Cells Galvanic cells harness the electrical energy available from the electron transfer in a redox reaction to perform useful electrical work. You will measure the cell potentials Ecell using a Vernier voltage probe as shown in Figure 3.
Galvanic cell is made up of two half cells ie anodic and cathodic. A galvanic cell is constructed by combining an oxidation electrode with a suitable reduction electrode to convert chemical energy into electrical energy by a redox reaction. The devices in which chemical reaction produces electrical energy are called electrochemical cells or galvanic cells or voltaic cells.
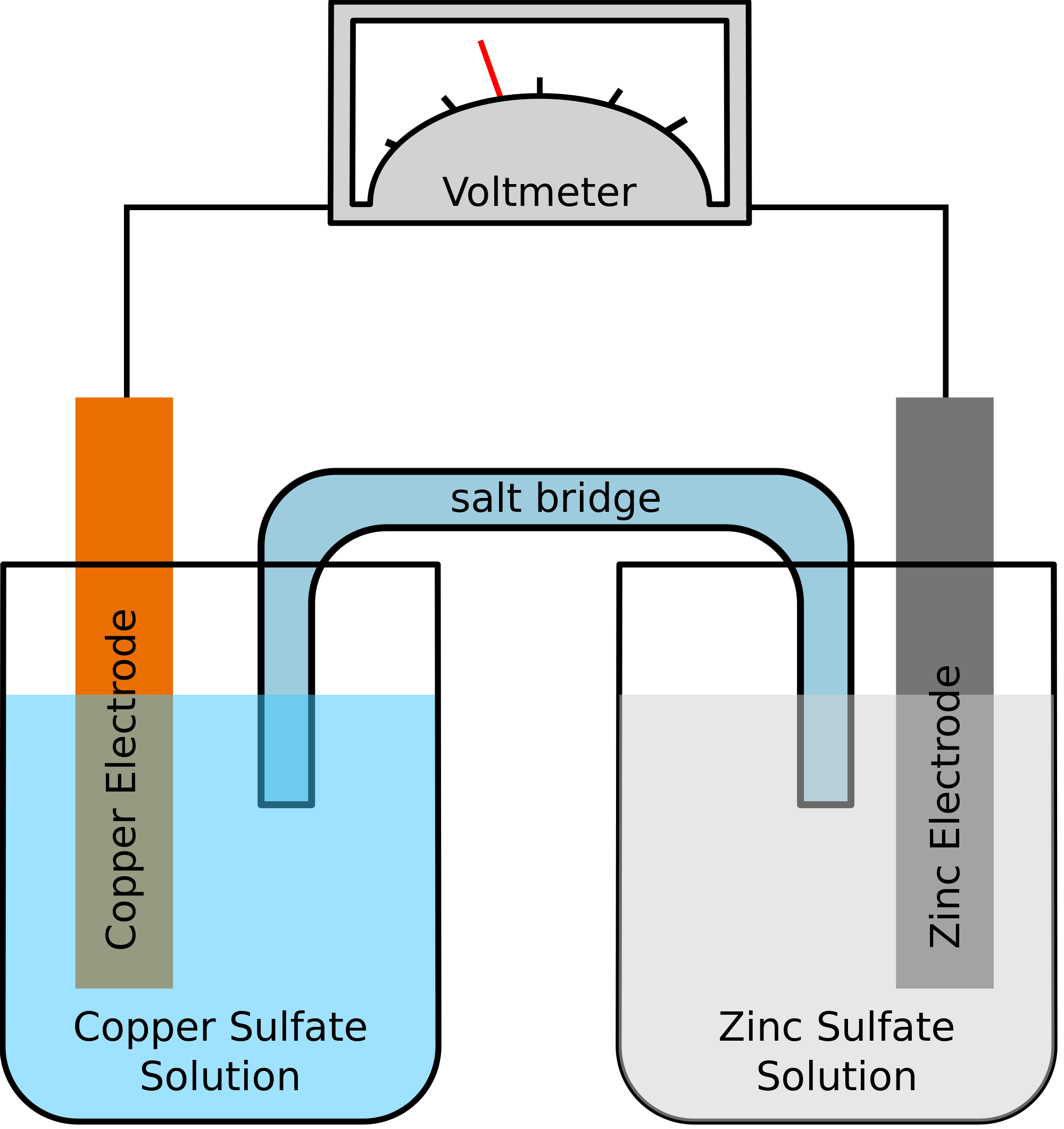
Consider a galvanic cell consisting of. Outline the construction of galvanic cells and trace the direction of electron flow. Zinc rod immersed in ZnSO4 behaves as anode and copper rod immersed in CuSO4 behaves as cathode.
You will use 10 M solutions for both half-cells so Q 1 and lnQ 0 for the reaction. The cell is comprised of two half-cells each containing the redox conjugate pair couple of a single reactant. For example a galvanic cell is created with the following two half-reactions.
The cell diagram can also be written using the. Oxidation takes place at anode and reduction at cathode. Displaystyle text 2Crleft sright text 3Cu 2left.
It is also known as voltaic cell. A galvanic cell based on the spontaneous reaction between copper and silver I is depicted in Figure 2. Two electrolytic solutions in which electrodes are immersed are connected to each through a porous diaphragm or a salt bridge.
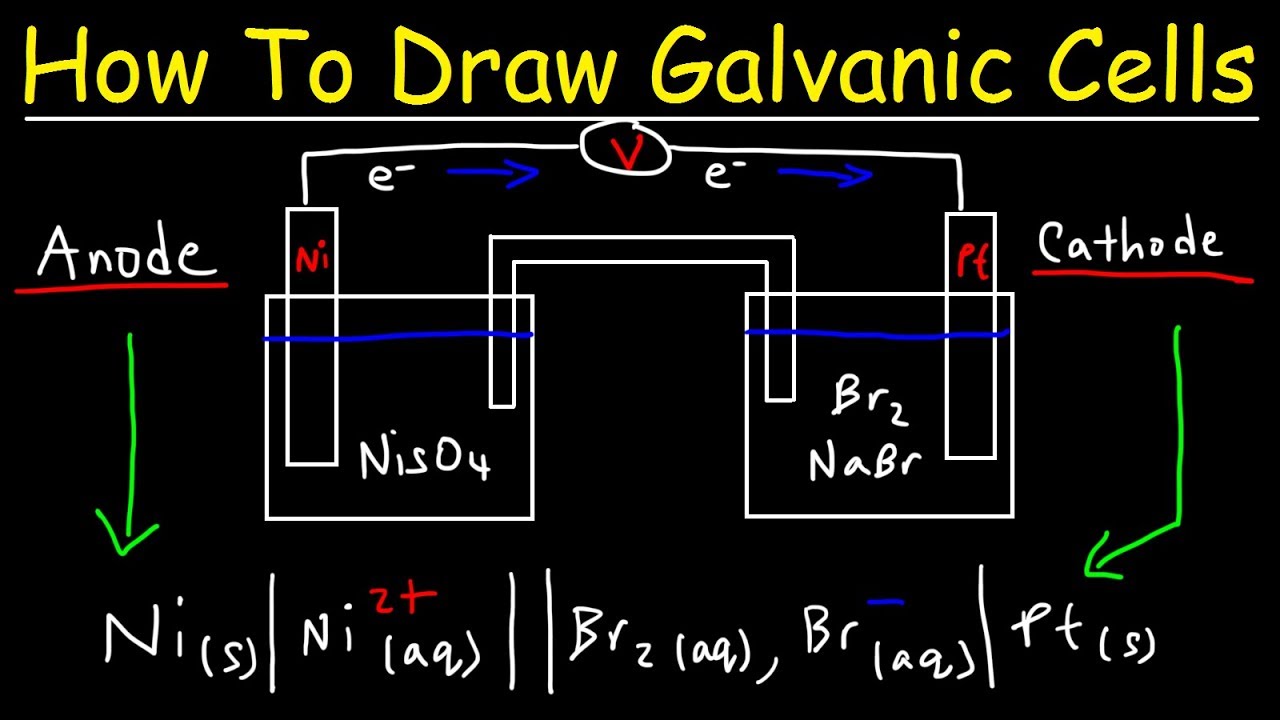
Electrochemical Methods. This chemistry video tutorial explains how to draw galvanic cells and voltaic cells given the overall reaction. Construct a galvanic cell using two metalmetal-ion half cells.
In writing the equations it is often convenient to separate the oxidation-reduction reactions into half-reactions to facilitate balancing the overall equation and to emphasize. The Galvanic Series also called the electro-potential series lists metals in the order of their nobility. DIY Battery Galvanic Cell Step 1.
Fe 3 a q e Fe 2 a q Zn 2 a q 2 e Zn s Now we want to determine what the standard cell potential E. In a galvanic cell Oxidation-Reduction chemical reactions produce electricity that can be used to turn on a flashlight lamp. From this you can quickly determine the anode oxidation and cathode reduction.
Describe using a diagram the essential components of a galvanic cell. Galvanic cells also known as voltaic cells are electrochemical cells in which spontaneous oxidation-reduction reactions produce electrical energy. The half-cell shown at the left contains the Cu 0Cu II couple in the form of a solid copper foil and an aqueous.
323 Galvanic Cells. You will then construct a series of three galvanic cells combining the zinc half-reaction with three different metal half-reactions Cu Fe and Pb. Setup of a Galvanic Cell.
The cell would ideally include two electrodes. You need this material. In this lab you will construct a galvanic cell using two metal electrodes and measure the potential produced as the oxidation and reduction reactions occur.
Measure the amount you calculated and transfer it. Production of Materials 4. First calculate the amount of copper sulfate that you will need to.
If we then substitute e- e-with the double vertical lines representing the separation of the two half-cells by the salt bridge we get. Dont forget to turn on Closed Captioning. Prepare 100 mL of a 005 M copper sulfate solution.
Galvanic cell is a device in which chemical energy is converted to electrical energy. It explains how to identify the anode and th. To begin put on the necessary personal protective equipment including a lab coat gloves and chemical splash goggles.
Calculate delta G standard for this reaction at 25 degrees celsius. In order to create a galvanic cell one would have to go through the following setup. Noble metals are those that are resistant to corrosion and oxidation When two metals are immersed in an electrolyte while also being connected externally by a conductor the less noble metal experiences galvanic corrosion.
Including the oxidation and reduction half-cells the positive and negative electrodes and their solutions of their ions the flow of electrons and the movement of ions and the salt bridge. The construction of a typical galvanic cell.
Galvanic Cells Working Setup Examples Terms Videos Q And A

Galvanic Cell With Zinc And Copper Galvanic Cell Electrochemistry Chemistry Lessons

Galvanic Cells Working Setup Examples Terms Videos Q And A

How To Draw Galvanic Cells And Voltaic Cells Electrochemistry Youtube
No comments for "How to Construct a Galvanic Cell"
Post a Comment